Hydroxyl radical (.OH) is the most reactive oxidant, which plays important roles in the biogeochemical cycle of elements and the attenuation of contaminants in the environment. In recent years, the redox reaction of iron in subsurface sediments was found to produce .OH naturally, even in dark conditions without the presence of exogenous hydrogen peroxide. However, the effect of mineral-associated soil organic matter (SOM) on the process are not well understood.
In a study published in Environmental Science & Technology, a research team led by Prof. ZHU Yongguan and A.P. LI Gang from the Institute of Urban Environment of the Chinese Academy of Sciences reported the influence of humic substances, the major components of SOM, on the microbially mediated iron reduction and reoxidation processes, and established the pathway of .OH production in different SOM-containing system.
The researchers used fulvic acid (FA), humic acid (HA), and humin (HM), components of humic substances operationally separated from soil, to evaluate the influence of SOM characteristics on iron redox processes. High electron exchange capacity of FA and HA promoted the microbial iron reduction process, while HA with high electron donating capacity inhibited the yield of .OH.
Using the scavengers of possible intermediate involved in .OH production, different pathways for .OH production in SOM-containing system were established. The one-electron transfer process dominated the .OH production in the FA-containing system, while both one- and two-electron transfer processes were present in HA- and HM-containing systems.
Microbially mediated iron redox processes changed the properties of dissolved fractions of SOM. The aromaticity of dissolved fraction of HA decreased due to its high reactivity with .OH.
Combined with the high resolution transmission electron microscope and X-ray diffractometer, ferrous secondary minerals formed and SOM inhibited its transformation to higher stable and crystalline iron oxy(hydr)oxides.
This work advances the understanding of SOM-involved iron redox processes and .OH production. The mechanisms revealed here need to be considered when evaluating the effect of potentially produced .OH on pollutants degradation in redox fluctuating environments.
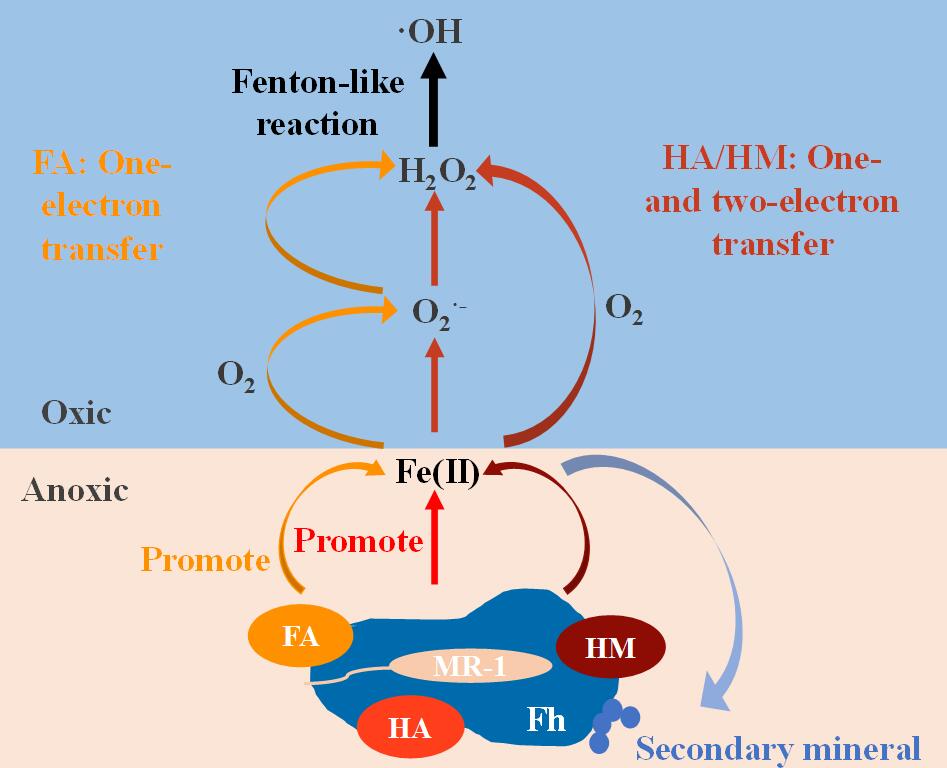
Figure 1. Mechanisms on ·OH production in microbial reduction-reoxidation process mediated by different organic matter components.
Page: Environ. Sci. Technol. 2022, 56, 22, 16419–16427
Contact: LI Gang Institute of Urban Environment, Chinese Academy of Sciences
E-mail: gli@iue.ac.cn