Recently, the Group of Environmental Functional Materials (Fu Lab) in the Institute of Urban Environment (IUE), CAS reported a new research work: Magnetic Mn-Fe oxycarbide can activate persulfate (PS) to form a diversity of reactive oxygen species (ROS), i.e., O2.-, 1O2, SO4.- and .OH, among which both O2.- and 1O2 can influence the formation of SO4.- and .OH, and SO4.- and .OH are the main ROS responsible for contaminant degradation. The work entitled “Novel magnetic rod-like Mn-Fe oxycarbide toward peroxymonosulfate activation for efficient oxidation of butyl paraben: Radical oxidation versus singlet oxygenation” has been published in the journal of Applied Catalysis B: Environmental. Dr. Jia-Cheng YANG was the first author of this work, and Prof. Ming-Lai FU was the corresponding author. The Key Laboratory of Urban Pollutant Conversion, CAS was the first corresponding address. This study was co-funded by the National Natural Science Foundation of China (Grant Nos. 51778598, 51808524 and 51478449), Fujian Provincial Key Laboratory of Ecology-Toxicological Effects & Control for Emerging Contaminants (PY18003), Science and Technology Major Project of the Bureau of Science and Technology of Xiamen (No. 3502Z20191012) and International Science & Technology Cooperation Program of China (2011DFB91710).
Over the past ten years, persulfate oxidation technique (POT) has been developed as a novel advanced oxidation technique for water treatment. However, the oxidation capacity of sole PS toward decontamination was proved to be limited. Activating PS by using metal oxides to generate SO4. (2.5–3.1 VNHE) can remarkably enhance the oxidation ability of PS. Compared to the classical heterogeneous Fenton oxidation technique, POT can be operated in a relatively wider pH range. With the ongoing study of POT consistently proceeded, developing activators/catalysts with high reactivity, robust structure/property and desirable reusability to generate the high oxidation capacity of ROS remains challenging. By virtue of its cost-effectiveness and controllable structure, Mn-based material (MNM) has been considered as a novel functional material toward new energy exploration and environmental remediation. It is generally accepted that MNM can activate PS to produce SO4..- and .OH radicals toward the degradation of refractory pollutants. Nevertheless, the resultant MNM prepared by various synthetic methods/procedures usually possesses differentiated structures and properties, thus affecting its efficacy toward PS activation; meanwhile, the nonradical oxidation process (i.e., 1O2 and electron shuttle mediated direct oxidation) has been discovered and has quickly become one of the most focused researches on POT. For example, α-MnO2 can activate peroxydisulfate to form 1O2 toward the selective removal of phenolic pollutants. Unfortunately, the information regarding the generation of a diversity of ROS in the MNM activated PS system and the related degradation mechanisms for contaminants still remains largely unknown.
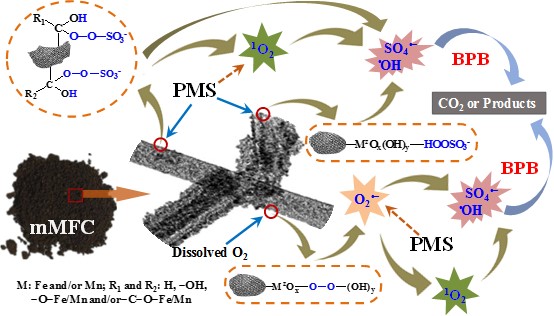
To this end, Fu Lab synthesize a rod-like Mn-Fe oxycarbide (mMFC) and studied its activation efficacy toward peroxymonosulfate (PMS) for degrading an emerging pollutant, butyl paraben (BPB), with the aim of probing the efficacy of such MNM toward PMS activation and the evolution mechanism of ROS. The resultant mMFC can be expressed as Fe24O32, in which the Fe in octahedral sites has been partially replaced by Mn (i.e., Mn,Fe)24O32/(Mn,Fe)3O4), thus exhibiting the similar crystalline structure of Fe24O32. The researchers used XPS, in situ ATR-IR and Raman to reveal the elemental/structural information of mMFC, and systematically analyzed the interactions between mMFC and PMS. The analysis of quenching tests and in situ EPR characterizations for the type of ROS indicated that the metal (Mn/Fe) and non-metal (C=O) sites within mMFC activated PMS synergistically to generate O2.-, 1O2, SO4.- and .OH. In addition, the researchers employed in situ EPR coupling with quenching tests, D2O experiments and theoretical calculation to analyze the ROS evolution and the contribution of ROS to BPB degradation. The results show that both O2.- and 1O2 affected the formation of SO4.- and .OH. SO4.- and .OH were identified as the main ROS responsible for BPB degradation, while O2.- and 1O2 served as a secondary driving force to form SO4.- and .OH. Acid etching experiments imply that the contributions of metal and non-metal sites within mMFC were 65.8% and 34.2% for PMS activation. This study uncovered a nonradical and radical process for pollutant degradation by novel MNM activated PS (see the following conceptual model), which would provide a more comprehensive and deeper understanding of the process and nature of contaminant removal by POT.