Infections caused by antibiotic resistant bacterial pathogens pose a fundamental threat to humans. They are predicted to surpass cancer and heart disease to become the leading cause of death in 2050. Speeding up antibiotic susceptibility testing (AST) is urgently needed in clinical settings to guide fast and tailored antibiotic prescription before treatment, not only curtailing medical cost, improving survival rate, but also providing an important way to control the occurrence and spread of antibiotic resistance. However, it remains a big challenge to achieve a sufficiently rapid sample-to-AST answer directly from a clinical sample within a quarter working day, due to the great challenges of complex clinical sample matrix and low cell loads. Current microbial growth-based standard AST assay used in clinical setting is very time consuming. It takes a lengthy 24 h-5 d for clinical sample precultivation and additional 16-24 h for AST. Very few methods can be directly performed on clinical samples.
The group led by Prof. Yong-Guan Zhu from Institute of Urban Environment, Chinese Academy of Sciences achieved a milestone for Raman spectroscopy-based rapid infectious disease diagnostics from clinical samples. They developed a rapid activity-based phenotypic AST approach directly applicable for clinical urine samples without the need of pre-cultivation via single-cell Raman spectroscopy coupled with heavy water labeling. The total assay time from receiving urine to binary susceptibility/resistance (S/R) readout was shortened to only 2.5 h, enabling a rapid diagnosis and timely guidance of antibiotic therapy for clinician (Figure 1). It well addressed the challenges of complex matrix of clinical samples and low cell counts by rapidly extracting and transferring bacteria in urine directly for AST within 15 min, much shorter than the 24 h for urine culturing. The ability of Raman spectroscopy to measure down to a single cell enabled the testing of limited number of bacteria in urines. Moreover, AST results from the developed Raman-heavy water assay achieved 100% categorical agreement with the slow gold standard AST assay used in clinical setting. This excellent consistence was achieved by optimizing antibiotic treatment condition to overcome the nonsynchronous responses between microbial activity and microbial growth, and also establishing a new S/R criterion for Raman-based AST to overcome the intrinsic activity variation of different bacteria. At least 7 antibiotics of different action mechanisms and 14 bacterial pathogens were demonstrated to work well with Raman-based AST assay, including Klebsiella variicola, Escherichia coli, Providencia rettgeri etc. The whole work flow was easy-handling, cost-effective and involved only easily accessible lab consumables. This work greatly promotes the new single-cell Raman-based AST assay towards clinical practicability and translation, which is of great interest to the field of infectious diseases and promotes antibiotic stewardship.
This work was mainly carried out by PhD student Kai Yang and Prof. Li Cui, and recently published in Analytical Chemistry (2019, 91, 6296-6303) with title of “Rapid Antibiotic Susceptibility Testing of Pathogenic Bacteria Using Heavy Water-Labeled Single-Cell Raman Spectroscopy in Clinical Samples”. This work was supported by National Natural Science Foundation of China.
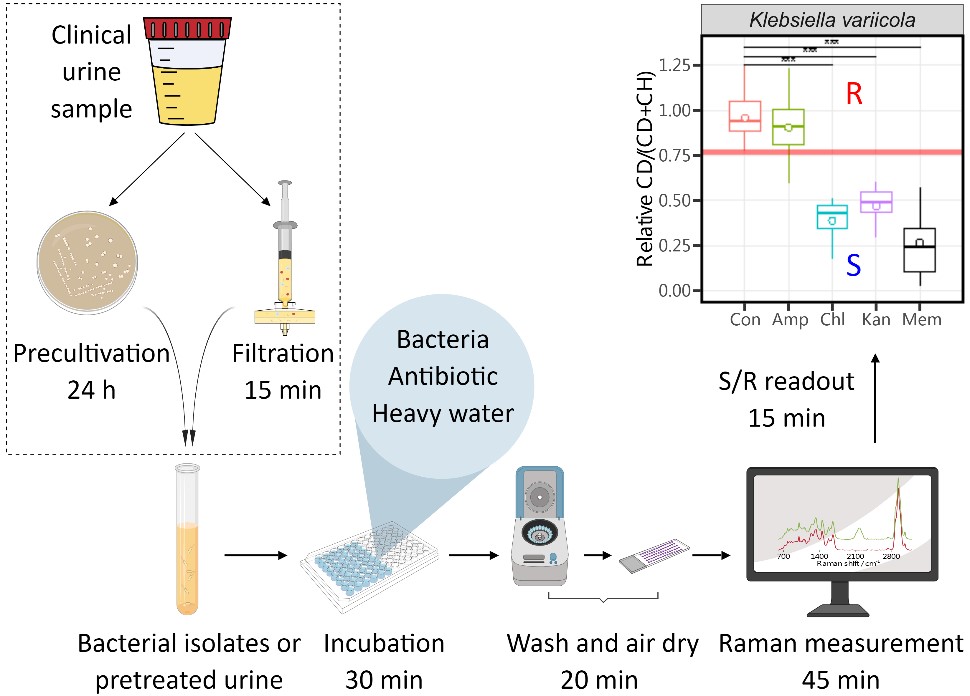
Figure 1. Workflow for antibiotic susceptibility testing via single-cell Raman-heavy water from clinical sample collection to susceptibility/resistance (S/R) readout. Pretreatment of urine via a rapid filtration or a lengthy precultivation on an agar plate are outlined by a dotted rectangle.