Volatile organic compounds (VOCs) emitted from anthropogenic and natural sources come into human living space and cause endanger to human health. Moreover, VOCs are major precursor leading to ozone and secondary organic aerosols (SOAs) in the atmosphere. Photocatalysis and catalytic oxidation are effective degradation methods for VOCs removal. However, for the degradation of aromatic compounds, such as toluene, they still suffer from low quantum efficiency and high energy consumption, respectively, which make it essential to find a good way that integrates multiple advantages to handle pollutants. Photothermocatalytic oxidation takes fully advantages of both UV-vis light with high energy and infrared light with thermal effect, whose application requires delicate design of photothermocatalysts and exploration of reaction mechanism.
Recently, a research team led by Prof. JIA Hongpeng from the Institute of Urban Environment of the Chinese Academy of Sciences has developed a manganese-based heterostructure composite catalyst and investigated the phoththermocatalytic oxidation reaction mechanism of toluene.
This study was published in Environmental Science & Technology on March 1.
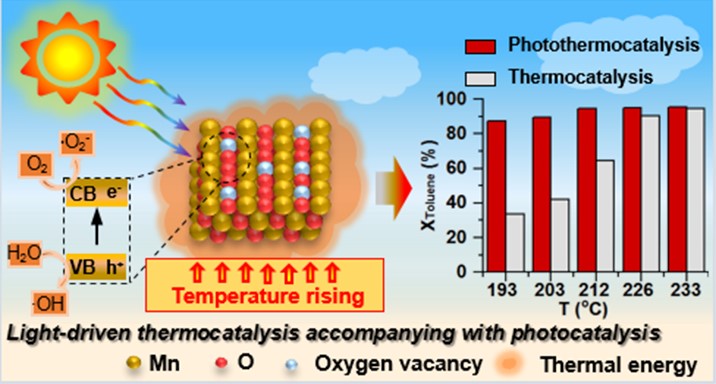
A series of experiments prove that strong light absorption capacity leads to highly utilization of light energy, and uniformly dispersed nanoparticles, large specific surface area, improved metal reducibility and oxygen desorption and migration ability at low temperature contribute to the advantageous catalytic performance of heterostructure catalyst compared with Mn2O3 under light irradiation. Photothermocatalytic oxidation exhibits better degradation efficiency at least 5 temperature points than conventional catalytic oxidation, and UV-vis and IR exhibit a comprehensive effect on promoting the photothermocatalytic activity. Light activated more lattice oxygen to participate in the reaction via Mars-van Krevelen (MvK) mechanism, and traditional e--h+ photocatalytic behavior exists over the heterostructure composite as an auxiliary degradation path.
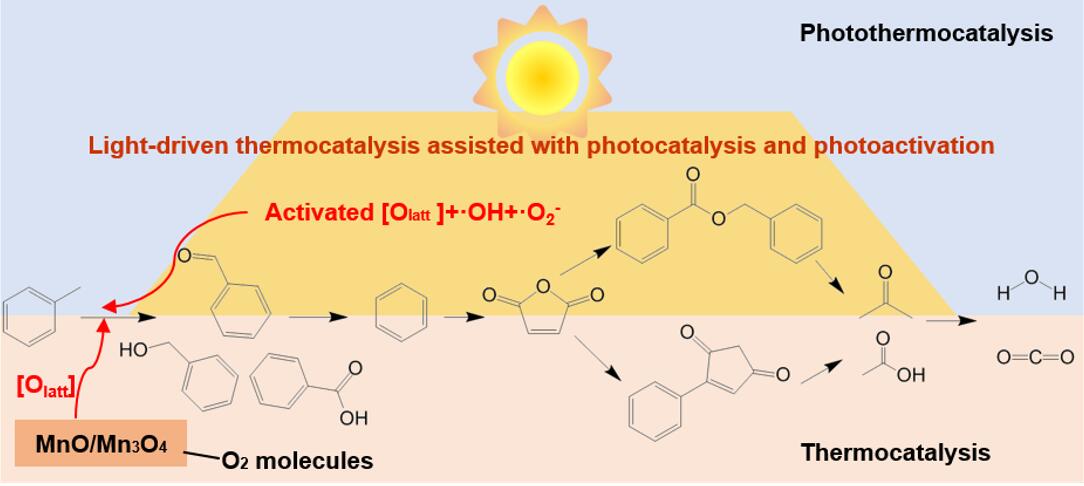
Overall, photothermocatalytic oxidation of toluene over manganese-based heterostructure composite catalyst is a photo-driven photothermocatalytic reaction, accompanied by the photoactivation of lattice oxygen and photocatalytic process, where lattice oxygen and reactive oxygen species (.O2– and .OH) are the main active oxygen species. The reaction pathways of photothermocatalysis and thermocatalysis are roughly the same (toluene → benzyl alcohol → benzaldehyde → benzoic acid → benzene → anhydride →acetone, acetic acid→ CO2, H2O), except the appearance of different copolymers (benzyl benzoate and phenylmaleic anhydride), where light enhances the deep conversion of intermediates. A proof-of-concept study under natural sunlight has confirmed the feasibility of practical application in photothermocatalytic degradation of pollutants.