Chlorinated organic compounds (CVOCs) represented by chlorobenzene are considered as hazardous pollutants, which not only are important precursors of extremely toxic dioxins, but also harm the ecological environment and human health. In recent years, efficient and harmless elimination of CVOCs has become a research hotspot. Among the numerous CVOCs elimination technologies, the catalytic oxidation technology with the development of suitable catalysts as the core has the advantages of simple operation and strong adaptability, and has the most application potential. Compared with ordinary organic pollutants, chlorinated organic molecules have a unique C-Cl bond, which can produce a large number of HCl and Cl2 components in the catalytic degradation process. They, which are easily adsorbed on the material surface and cause catalyst inactivation. Moreover, the presence of highly reactive Cl2 will also lead to the generation of highly toxic polychlorinated by-products. Therefore, the development of industrial catalysts with high chlorine resistance and low Cl2 selectivity is the key to the efficient and harmless elimination of CVOCs.
In the report of Prof. JIA Hongpeng and his team at the Institute of Urban Environment, Chinese Academy of Sciences, a strong acidic catalyst S-Ce0.7Zr0.3O2 was constructed by introducing Lewis and Bronsted acid sites on the surface of cerium oxide catalyst through zirconium ionizing phase doping and sulfur surface modification. The study showed that the introduction of two types of acid sites can effectively improve the activity and stability of chlorobenzene degradation on the cerium oxide. The introduction of Lewis acid sites can promote the activation of lattice oxygen and enhance the low temperature reducibility of catalyst, and is conducive to the oxidation of organic compounds and the removal of adsorbed chlorine. And the Bronsted acid sites can change the degradation path of chlorobenzene from oxidation route to hydrolysis route, promote the emission of Cl poisoning species in the form of HCl, fundamentally inhibited the generation of Cl2, thus reducing the selectivity of chlorinated by-products and Cl2 (only 1.2%). Because of the synergistic effect of Lewis and Bronsted acid sites, S-Ce0.7Zr0.3O2 showed excellent catalytic performance, achieving 90% chlorobenzene conversion at 406 °C and maintaining the same activity over a long period of 106 h.
Related findings were published in “Catalysis Science & Technology” with title of “Tuning the degradation activity and pathways of chlorinated organic pollutants over CeO2catalyst with acid sites: synergistic effect of Lewis and Bronsted acid sites” . PhD student LV Xuelong is the first author of this paper, and Professor JIA Hongpeng is the corresponding author. This work was supported by the National Key R&D Program of China (2019YFC1806102); the National Nature Science Foundation of China (21976172); and the “Key Research Program of Frontier Sciences” from Chinese Academy of Sciences (QYZDB-SSW-DQC022).
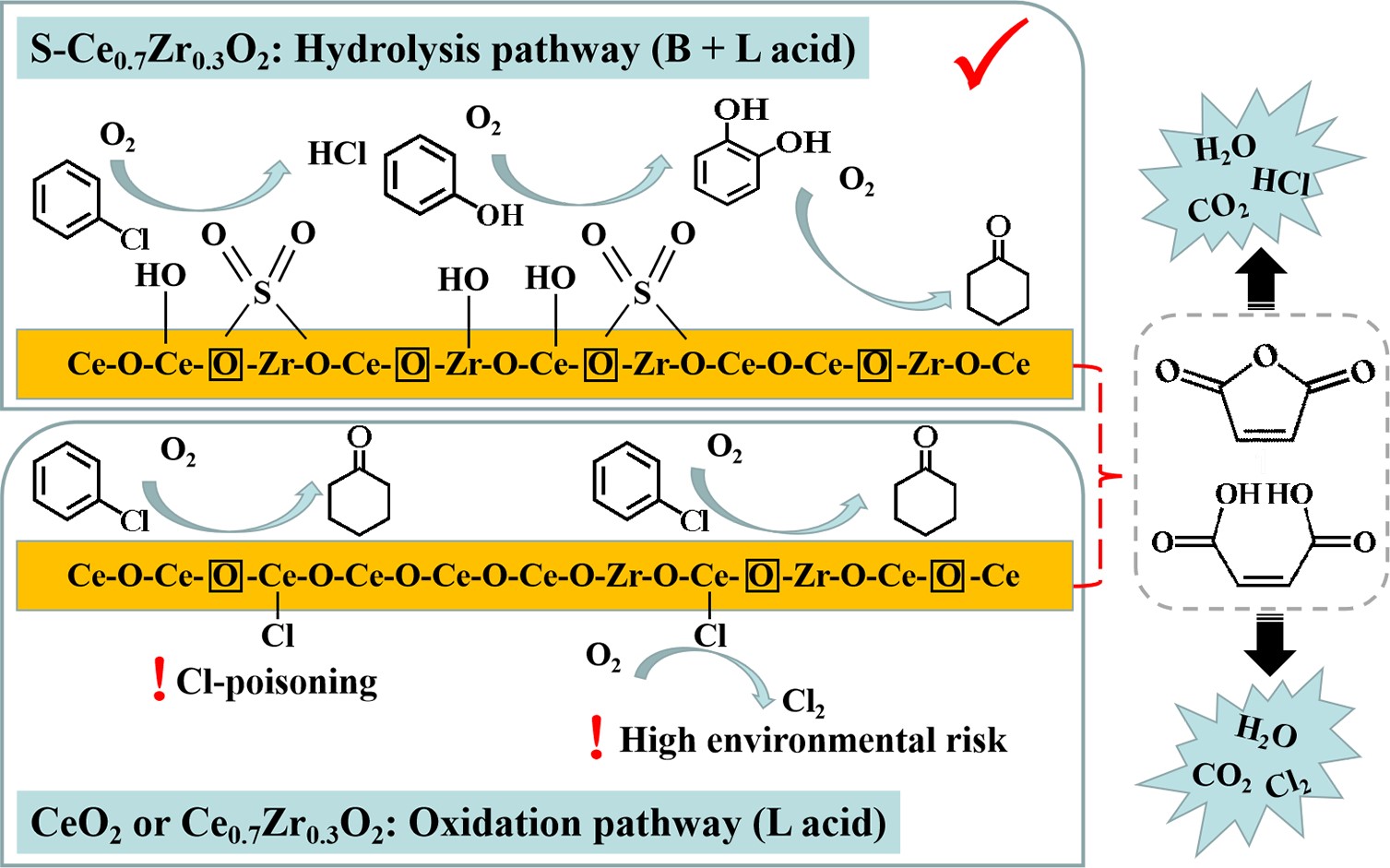
Regulatory mechanisms of Lewis and Bronsted acid sites on chlorobenzene degradation