Biosynthesis based on the reducing capacity of electrochemically active bacteria is frequently used in the reduction of metal ions into nanoparticles as an eco-friendly way to recycle metal resources. However, those bionanoparticles cannot be used directly as electrocatalysts because of the poor conductivity of cell substrates. This problem was solved by a hydrothermal reaction, which also contributes to the heteroatom doping and alloying between Pd and Au. With the protection of graphene, the aggregation of nanoparticles was successfully avoided, and the porous structure was maintained, resulting in better electrocatalytic activity and durability than commercial Pd/C under both alkaline (CH3CH2OH, 6.15-fold of mass activity) and acidic (HCOOH, 6.58-fold of mass activity) conditions. The strategy developed in this work opens up a horizon into designing electrocatalysts through fully utilizing the abundant resources in nature.
This work has been done by the research group of Environmental Biolelectrochemistry (Zhao Feng’s Group) in IUE, CAS and Han Heyou’s Group in Huazhong Agricultural University. The research was supported by NSFC (21375043, 21175051, 21322703), and published in Science Advances (Microbial synthesis of highly dispersed PdAu alloy for enhanced electrocatalysis, 2016, 2, e1600858; http://advances.sciencemag.org/content/2/9/e1600858).
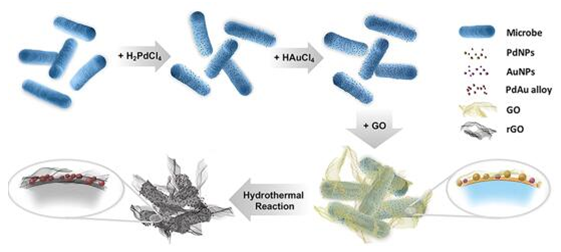
Figure 1. Formation mechanism for DPARH